A process to access selectively 1,4-diamines with two stereogenic centers that can aid in identifying new drug compounds
Unmet Need
When developing new drugs, researchers often rely on naturally occurring compounds to learn what design features illicit a biological response that can be tailored for treating a given disease or ailment. However, reproducing these natural compound features in a lab is incredibly challenging. For example, the 1,4-diamines motif is pervasive in naturally occurring compounds and is known to be bioactive as evidenced by pyrrolidines, one of the most commonly used heterocycles observed in approved drug compounds. However, our current access to 1,4-diamine molecules that we can build into promising drug candidates to take advantage of this bioactivity is very limited. There is a need for new reactions and advanced intermediates that expand the scope of biologically active structures that can be reproduced in a lab.
Technology
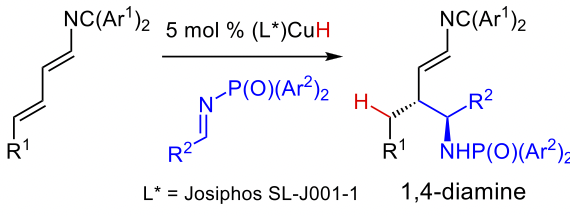
Duke inventors have developed a process for creating useful diamine chemical compounds that are currently difficult or impossible to access. These enantioenriched advanced intermediates are useful motifs for potential active drug compounds and would be valuable to include in fragment drug screening assays. Specifically, this is a method to enantioselectively, diastereoselectively and regioselectively prepare 1,4-diamines with two stereogenic centers. The inventors have demonstrated this process using 2-azatrienes, N-phosphinoyl imines, and the SL-J001-1 Josiphos ligand.
Advantages
- Offers an efficient and selective route to a bioactive motif, 1,4-diamines, that can be easily built upon to access lead compounds for drug candidates and other applications
- Through the reductive amination of aldehydes, this process can lead to highly substituted pyrrolidines
- Introduces an underutilized reagent class, 2-azatrienes, to achieve enantioselective synthesis of allylic amines